-
 • 11/17/23
• 11/17/23Expanding Technical Documentation Capabilities
In this webinar, JANA’s Don Bridges and JANA Life Science’s Ron Niland give a high-level overview of how your company can benefit from working with a technical documentation services vendor. The insight that they share will provide you with a set of ground rules that you will need to be aware of as you set out to establish a working relationship with a vendor, and they finish up by answering a few questions from attendees.
-
 • 6/21/23
• 6/21/23(1/7) Current State of Best Practices & Technology for Life Sciences - DCL Learning Series Webinar
This is the first webinar in a series of informational and interactive presentations for the life sciences industry. This collaborative webinar is put together from three leading technology companies---Data Conversion Laboratory, Court Square Group, and Jana Life Sciences. Each organization highlights an aspect of technology critical to improving how the biomedical and pharma industries can increase business performance while also securing intellectual property.
-
 • 6/21/23
• 6/21/23(2/7) Metadata & Taxonomies in the Drug Development Lifecycle
(60 minutes) Traceability, compliance, and shortening time to market – all of these business necessities are hot topics right now, and accuracy is a critical element to properly managing each one. A key to building efficiency into your processes and improving your content management strategies is establishing and maintaining a sound metadata and taxonomy strategy.
Along with Data Conversion Laboratory and Court Square Group, JANA Life Sciences presents this second segment in a learning series which will share with you ways that you can streamline R&D efforts and improve time-to-market by evaluating your content development and data management efforts – and even your IT systems – and ensure that each one of these program elements is a contributing factor to your success.
So if creating a metadata and taxonomy strategy is of interest to you, sit back, click the button and enjoy: Metadata and Taxonomies in the Drug Development Lifecycle (why should I care), brought to you by JANA Life Sciences, Data Conversion Laboratory and Court Square Group. -
• 6/21/23
(3/7) Improving Time to Market for Drug Development: Content Structure and Systems Integration
(60 minutes) When looking to improve drug development time-to-market, effective system and data flows are critical to ensuring your content is properly structured for interoperability. Content lifecycle management, including the use of automation, is key to the efficient flow of information between the various groups and systems involved.
This third segment in our 7-part webinar series will show you how well-structured data, content, and communications can provide the functional framework necessary for timely drug development.
If streamlining your drug development efforts through improving data management, IT systems, and regulatory compliance is of interest to you, we welcome you to sit back and enjoy the conversation around: Improving Time to Market for Drug Development: Content Structure and Systems Integration, brought to you by JANA Life Sciences, Data Conversion Laboratory, and Court Square Group. -
 • 6/21/23
• 6/21/23(4/7) Life Sciences IT Systems: Cloud, Compliance, Regulatory–All Together Now
(60 minutes) Moving from a closed internal system to a cloud-based system requires the ability to understand the support model from external vendors instead of relying on internal IT resources. Reconciling multiple cloud environments and how to most effectively use them while ensuring secure access is what enables successful interoperability.
We’ll dive deep into various scenarios, and what’s critical to understand including:
• Hybrid approach to internal and external systems
• Multi-cloud implementations
• Qualifying and validating applications in the cloud
• Migrating data between systems
• Information models in an inter-connected, cloud-based environment
• Taxonomy management across collaborators
• Content normalization and semantic enrichment
PANELISTS
Ron Niland, President, JANA Life Sciences
Keith Parent, CEO, Court Square Group
Tammy Bilitzky, CIO, Data Conversion Laboratory
This webinar is the fourth in a series to inform Life Science organizations about technology considerations related to the creation and management of content and technical data.
-
 • 6/21/23
• 6/21/23(5/7) Content From Multiple Clinical Sources - DCL Learning Series Webinar
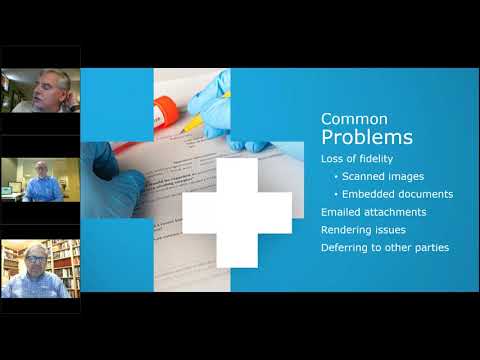
(60 minutes) Sponsors use many external vendors including CROs and multiple clinical sites during clinical trials. Based on the technical fluency of the users at these sites the documents and content that sponsors receive back may not be as usable as they could be. How do we put concepts in place that enable us to have a more consistent level of quality in documents?
This webinar informs content structure details that are often misunderstood and have significant impact on interoperability:
– Scanned images and the difference between Image files and OCRed content
– Standard formatting for ease of consolidation
– Content mapping to generate summary data
PANELISTS
Mark Gross, President, Data Conversion Laboratory
Keith Parent, CEO, Court Square Group
Ron Niland, President, JANA Life Sciences
This webinar is the fifth in a series to inform Life Science organizations about technology considerations related to the creation and management of content and technical data. -
 • 6/21/23
• 6/21/23(6/7) Technology & Life Sciences: Tool Selection, Systems Implementation, Data Migration, & Security
(60 minutes) The Life Sciences industry has always been concerned with security and regulatory compliance, particularly when implementing any new technology related to IT systems or content management.
Yet when looking at the drug development life cycle, new tools and processes can become vital components for handling the ever growing amount of data necessary to take a drug to market.
This webinar will identify a number of tools used successfully in other industries, tools that have evolved to meet the needs uniquely specific to the Life Sciences.
– RPA (Robotic Process Automation)
– AI (Artificial Intelligence) and ML (Machine Learning)
– Voice, video and image processing in clinical settings
PANELISTS
David Turner, Consultant, Data Conversion Laboratory
Keith Parent, CEO, Court Square Group
Ron Niland, President, JANA Life Sciences
This webinar is the sixth in a series of seven webinars to inform Life Science organizations about technology considerations related to the creation and management of content and technical data. -
 • 6/21/23
• 6/21/23(7/7) Map or Scrap? Consolidating Data Between Inherited Enterprise Systems in the Life Sciences
(60 Minutes) Mergers and acquisitions often involve many moving parts. One of those parts is an organization’s enterprise content management systems and how to reconcile multiple systems into a single one.
Best practices suggest consolidating multiple systems into one. This approach forces organizations to: conduct a content audit, harmonize duplicate content, and normalize content structures and metadata.
Join us as we cover the following topics and help ease the burden of what seems (but is not) an insurmountable task:
– Running two systems simultaneously: what are the pitfalls?
– Integration with other enterprise systems
– Competing metadata and naming systems
PANELISTS
Mike Gross, President, Data Conversion Laboratory
Keith Parent, CEO, Court Square Group
Ron Niland, President, JANA Life Sciences
This webinar is the seventh in a series to inform Life Science organizations about technology considerations related to the creation and management of content and technical data.
